Challenges of Acquiring Patient iPSC Lines
The Power of Patient iPSC Lines for Drug Discovery
The revolutionary breakthrough of Induced Pluripotent Stem Cells (iPSCs) has opened exciting new avenues in regenerative medicine and drug discovery. Among the various applications, patient-specific iPSC lines offer immense potential for personalized medicine and disease modeling, allowing researchers to study neurodegenerative diseases and other conditions in a more relevant and patient-specific context. However, obtaining patient iPSC lines for research purposes presents a unique set of challenges. In this article, we will delve into the obstacles that researchers may encounter during the acquisition of patient iPSC lines and explore case studies based on real experiences from Elixirgen Scientific.
What Researchers Need to Know Before Beginning to Use Patient iPSCs
Appropriate Intellectual Property Licenses:
Many iPSC lines may be protected by patents or other proprietary rights. Prior to initiating the acquisition process for patient iPSC lines, researchers must ensure that they hold the necessary intellectual property licenses. Failure to secure the appropriate licenses could lead to legal complications and impede the progress of research projects. It is essential to verify the licensing status of the iPSC lines and carefully review the material transfer agreements to avoid any potential legal liabilities.
Your Institutional Review Board (IRB) Requirements:
Ethical considerations play a paramount role when using patient-derived cells. Researchers must adhere to the guidelines set by their Institutional Review Board and ensure appropriate informed consent was obtained from patients before acquiring and using iPSC lines. For instance, certain IRBs may impose restrictions on the use of biological samples from compensated donors, prohibiting their use for research purposes. Adhering to these ethical guidelines ensures responsible research conduct and upholds patient privacy and autonomy.
Operating Body of iPSC Line Provider:
Selecting a reliable and reputable iPSC line provider is of utmost importance. Researchers should thoroughly investigate the operating body behind the iPSC bank or repository. iPSC banks can be operated by for-profit companies, academic institutions, or non-profit organizations, each having its pros and cons. Familiarity with the provider's background, track record, and data sharing policies empowers researchers to make informed decisions. It is important to be aware that some iPSC banks may exhibit slow responsiveness or may not respond at all, hindering the acquisition process. A recent endeavor of one of our clients took nearly a year from the initiation of the order to the actual receipt of the lines. It's essential to exhibit patience and factor potential delays into your project timeline.
Understanding of the Provider Requirements:
Certain iPSC banks may have restrictions on providing iPSC lines to for-profit organizations. Awareness of the specific requirements and restrictions imposed by the provider is crucial for a seamless acquisition process. Clear communication with the provider and thorough comprehension of their requirements will prevent misunderstandings and potential delays in obtaining the iPSC lines. For instance, in theory, all commercial entities should possess an iPS Academia Japan license even for in-house research. Nevertheless, this requirement may vary as some banks mandate it, while others do not.
Variances in iPSC Line Quality:
The quality of iPSC lines can profoundly impact research outcomes. Researchers must consider factors such as the source of somatic cells used for reprogramming, the reprogramming method, and the validation procedures employed by the iPSC line provider. Variability in cell quality can influence experimental reproducibility and hinder the generation of reliable data. For example, Elixirgen Scientific has encountered multiple iPSC lines contaminated with differentiated cells, necessitating the selection of appropriate iPSC colonies for further differentiation projects. Additionally, some iPSC banks may not provide information on the growth rate of the iPSC lines, which is crucial in assessing yield from iPSCs to differentiated cells, highlighting the need for standardized growth rate measurement.
Case Studies from Elixirgen Scientific’s Experience
1. Fast Turnaround with For-Profit Company Operated iPSC Bank:
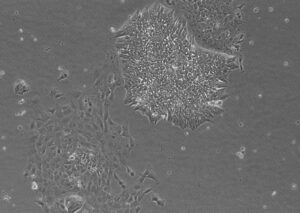
Elixirgen Scientific had successful experiences acquiring patient iPSC lines from a reputable for-profit company operated iPSC bank. The iPSC lines were quickly acquired and provided a streamlined route from culture generation, harvesting, and effective differentiation through our patented process. However, despite the quick turnaround time, we found up to 20% of received iPSC lines contaminated with differentiated cells after initial thawing (see image below). Such a situation required iPSC colony picking for multiple passages. Even if you receive a new vial, it may be similarly contaminated and requires additional workload.
2. Stricter Document Requirements:
In another case, Elixirgen Scientific encountered challenges when dealing with a provider with stringent document requirements. While ensuring proper compliance is essential, overly burdensome paperwork can cause delays in the acquisition process and impede research progress.
3. Canceled Order with Cross-Border iPSC Procurement:
Cross-border procurement of patient iPSC lines can introduce unforeseen complications. Elixirgen Scientific faced a slow response and eventually canceled an order due to inconsistent messaging and communication issues. Researchers must be prepared for potential complexities when acquiring iPSC lines from international sources, and clear communication channels with the iPSC bank or its distributor are crucial. Unfortunately researchers cannot always believe and use provided information on websites. There could be logistical challenges and varying degrees of customer support.
4. Blind Order with a Non-Profit Institute Based iPSC Bank:
Elixirgen Scientific’s client encountered communication challenges with a non-profit institute-based iPSC bank, resulting in an unclear order requirement and prolonged delays. After some time, it became clear that the bank subjectively chooses who to provide iPSC lines to, even when their disclosed Material Transfer Agreement (MTA) does not require any special conditions. Unfortunately, the institute's interests may supersede enabling wider industry use, impacting researchers' access to the iPSC lines.
Potential alternatives to the procurement from iPSC banks
Gene-Edited iPSCs as an Alternative to Patient iPSC Lines
Considering the challenges associated with acquiring patient-specific iPSC lines, researchers have explored gene-edited iPSCs as a viable alternative. Leveraging gene editing technologies such as CRISPR-Cas9, researchers can introduce disease-specific mutations into healthy iPSC lines, creating disease models that closely resemble patient-specific iPSCs. While these gene-edited iPSCs may not entirely replicate the patient's genetic background, they offer valuable insights into disease mechanisms and potential therapeutic targets. Also, for those looking for more relevant controls, using gene-editing to create wild-type or corrected rescues for an isogenic control can help.
Viral Transduction to Introduce Target Disease State
Another alternative researchers could consider is engineering existing cell lines. Elixirgen Scientific's iPSC-differentiated neurons are highly viable and can be transduced with lentivirus to introduce disease-like states. For instance, GFP-tagged lentiviral transductions of Elixirgen Scientific neurons have been successfully demonstrated after thawing, providing researchers with a flexible and effective tool for studying disease mechanisms.
Conclusion
Patient-specific iPSC lines serve as a potent resource for advancing drug discovery and personalized medicine, especially in disease modeling and iPSC drug screening applications. However, researchers must be aware of the challenges involved in acquiring these valuable resources. Ensuring proper compliance with intellectual property licenses and ethical guidelines, selecting reputable providers, and considering alternatives like gene-edited iPSCs and viral transduction will contribute to a more successful and impactful iPSC-based research journey. Collaborative efforts among iPSC banks, researchers, and ethical governing bodies will unlock the full potential of patient iPSC lines, fostering transformative medical advancements in neurodegenerative diseases and beyond.
Contact us
List of diseases and mutations.
Transform how you study disease with Quick-Tissue™ technology
With Quick-Tissue™ technology, you can study disease state from multiple angles in vitro. Thanks to advance in iPSC reprogramming technology, there are thousands of iPSC lines available for your disease study. Look through the iPSC line database below to find out your diseases in interest exist. The database also lists identified mutant genes. Feel free to reach us to how we can help getting differentiated cells derived from iPSC lines in your interest.
Disease Mutant genes (# of iPSC lines) Number of total patient iPSC lines
ABCA1 heterozygous ABC1 (2) 2
Abetalipoproteinemia MTP (2) 2
Acromesomelic dysplasia NPR2 (1) 1
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) 1
Adrenoleukodystrophy (ALD) 1
Adult-onset Still’s disease (AOSD) 1
Age-related macular degeneration (AMD) 121
Aicardi syndrome 1
Alexander disease GFAP (3) 6
Allergic granulomatous angiitis 1
Alzheimer's disease (AD) APOE (10), APOE4 (3), APP (4), C9ORF (1), CD33 (2), MAPT (2), PSEN1 (14), PSEN2 (1), TBK1 (1), TREM2 (3) 180 (Available Differentiated cells)
Alzheimer's disease (AD) (Gene-edited) APP (6), PSEN1 (8) 14
Alzheimer's disease (AD) (familial) APP (3), APPV7171 (4), PSEN1 (1), PSEN2 (1) 11
Amyotrophic lateral sclerosis (ALS) ASYMPTOMATIC C9ORF72 CARRIER (1), C9ORF72 (46), FIG4 (1), FUS (3), SETX (1), SETX, SOD1 (1), SOD1 (36), SOD1 > D90A (1), TARDBP (5), VCP (1) 532
Anemia (phenotype) 1
Angelman syndrome UBE3A (2) 10
Aplastic anemia 3
Arrhythmogenic right ventricular cardiomyopathy 2
Arteriolosclerosis 2
Associated pulmonary arterial hypertension 18
Ataxia-telangiectasia 3
Atrial fibrillation 14
Atrial tachycardia 1
Autism spectrum disorder (ASD) 110 (Available Differentiated cells)
Autoimmune hemolytic anemia (AHA) / Idiopathic warm (AHA) 1
Bardet-biedl syndrome 22
Batten disease (cln3) CLN3 (23) 23
Batten disease (cln6) 8
Behçet’s disease 2
Beta thalassemia HBB (2) 2
Bethlem myopathy 2
Bilateral frontoparietal polymicrogyria GPR56 (1) 1
Bipolar disorder 30
Blinding eye disease 18
Borderline NASH (fatty liver disease) 2
Breast cancer BRCA1 (3) 3
Brugada syndrome 6
Buerger’s disease 1
Cardiomyopathy 48
Carpal tunnel syndrome 18
Catecholaminergic polymorphic ventricular tachycardia RYR2 (2) 2
Ccanavan Disease ASPA (1) 1
Cchoroideremia (CHM) CHM (1), NGLY1 (1) 4
Cerebral creatine deficiency syndrome 1 (CCDS1) SLC6A8 (1) 1
Cerebral palsy (CP) 19
Cerebrovascular disease 4
Ceroid lipofuscinosis CHM (1), CLN2 (1) 2
Charcot-Marie-Tooth disease FIG4 (1), MFN2 (10), MPZ (2), PMP22 (7), VCP (1) 22
Chromosome 16p11.2 deletion syndrome 5
Chronic inflammatory demyelinating polyneuropathy (CIDP) 2
Chronic myeloid leukemia 1
Congenital disorder of deglycosylation (CDDG) CFTR (1) 1
Congenital heart block 2
Congenital ichthyosis / Ichthyosis syndrome 1
Congenital insensitivity to pain with anhidrosis (CIPA) 2
Congenital myasthenic syndrome GFPT1 (1) 7
Congenital myopathy MTM11 (1) 1
Control C9ORF72 (5), CCR5 (1), GFAP CORRECTED (2), HBB (1), HD (5), HNF1A (1), MECP2 (2), NGN2 (2), SNCA (1), SOD1 > D90A CORRECTED (1), TAF1 VARIANT CORRECTED (4) 25
Coronary artery disease 43
Corticobasal degeneration (CBD) 1
Crohn's disease 3
Crow‐Fukase syndrome 2
Cystic Fibrosis (CF) DMPK (1) 1
Cystinosis 1
DMD DMD (5) 5
DMRV / GNE myopathy 2
Danon disease 1
Definite NASH (fatty liver disease) 32
Diabetes HNF1A (2) 3
Diabetes mellitus 60
Diabetes mellitus type II 12
Diabetes type I 21
Diabetes type II 95
Diabetes type unknown 4
Diabetic retinopathy (DR) 32
Diamond-Blackfan anemia 1
Dilated cardiomyopathy (DCM) 303
Distal Myopathy 3
Down syndrome 47,XX,+21 (4), 47,XY,+21 (3) 8
Dravet syndrome SCN1A (11) 11
Drug-induced liver injury (DILI) 4
Duchenne Muscular dystrophy (DMD) DMD (1) 2
Dystrophia myotonica 1 (DM1) DMPK (1) 1
Ehlers-Danlos syndrome COL3A1 (1) 2
Eosinophilic granulomatosis with polyangiitis (EGPA) 1
Eosinophilic sinusitis 1
Epidermolysis bullosa 1
Epilepsy ALG13 (1), GABRA1 (1), KCNC1 (1), PCDH19 (2), SCN2A (1) 57 (Available Differentiated cells)
Fabry disease 3
Facioscapulohumeral muscular dystrophy 1 (FSHD1) LRIF1 (1) 6
Familial Mediterranean fever 1
Fatty liver disease - steatosis (not NASH) 2
Focal cortical dysplasia (FCD) 2
Focal segmental glomerulosclerosis 20
Fragile X syndrome FMR1 (4) 4
Friedreich ataxia 1 (FRDA) FXN (2) 2
Frontotemporal degeneration C9ORF72 (4), GRN (4), MAPT (10), PGRN (2), VCP (1) 25
Frontotemporal dementia (FTD) C9ORF72 (6), MAPT (15) 30
Frontotemporal lobar degeneration (FTLD) 2
GM1-gangliosidosis 1
Galactosialidosis 1
Gaucher disease GBA (1) 2
Giant cell arteritis (GCA) 2
Glaucoma 20
Glut1 deficiency syndrome 1 (Glut1DS1) SLC2A1 (1) 1
Glycogen storage disease / GSD type V (muscle glycogen phosphorylase deficiency) 1
Glycosylphosphatidylinositol(GPI) anchor deficiency 3
Granulomatosis with polyangiitis (GPA) 1
Hemiconvulsion-hemiplegia-epilepsy syndrome 2
Hemimegalencephaly 1
Hepatitis C (HCV) 91
Hereditary dystonia 1
Homozygous familial hypercholesterolemia LDLR (6) 6
Hunter syndrome 2
Huntington's disease (HD) HD (14), HTT (36), IT15 (1), SMN1 (1) 58
Hurler syndrome IDUA (1) 1
Hutchinson-gilford progeria syndrome (HGPS) 2
Hyperalphalipoproteinemia SR-BI (2) 2
Hypertrophic cardiomyopathy TNNT2 (1) 83
Idiopathic aplastic anemia 1
Idiopathic pulmonary arterial hypertension 16
Idiopathic pulmonary fibrosis (IPF) 182
Idiopathic thrombocytopenic purpura 1
IgG4-related disease 1
IgG4-related thyroid disease 1
Inappropriate sinus tachycardia 1
Infantile neuroaxonal dystrophy (INAD) PLA2G6 (5) 5
Intellectual disability (ID) 60
Interstitial lung disease 1
Isaacs syndrome 1
Isogenic control 3
Kearns-sayre syndrome (KSS) 1
Keratoconus 2
Krabbe disease GALC (1) 1
Landau-Kleffner syndrome 1
Left ventricular hypertrophy 15
Left ventricular non-compaction cardiomyopathy 10
Lennox-Gastaut syndrome (LGS) 2
Lesch-nyhan syndrome (LNS) HPRT1 (1) 1
Lewy body dementia 8
Lewy body dementia (LBD) 1
Limb-girdle muscular dystrophy DYSF (5) 5
Limb-girdle muscular dystrophy (LGMD2b) DYSF (15) 15
Lissencephaly DCX (1) 1
Long QT syndrome 3
Long QT syndrome (familial) 13
Long QT syndrome 1 (LQT1) 3
Long QT syndrome 2 (LQT2) KCNH2 (1) 1
Long QT syndrome 3 (LQT3) SCN5A (1) 1
MELAS 1
Malignant rheumatoid arthritis (MRA) 1
Mental illness DISC1 EXON 8 WILD-TYPE (2) 2
Mental retardation CHAMP1 (2), SYNGAP1 (1) 3
Mesial temporal lobe epilepsy with hippocampal sclerosis 2
Microscopic polyangiitis (MPA) 1
Migraine disorder MAJOR CHR17 AMPLIFCATION; MINOR CHR7 DELETION (1) 28
Mild left ventricular hypertrophy 1
Miller-dieker lissencephaly syndrome (MDLS) 1
Mitochondrial diseases 1
Mixed Connective-Tissue Disease (MCTD) 1
Monogenic diabetes 13
Mortor dominant 1
Moyamoya disease 3
Mucopolysaccharidosis (MPS) SGSH (1) 3
Multifocal motor neuropathy (MMN) 1
Multiple sclerosis (MS) 4
Multiple system atrophy (MSA) 6
Muro disease (Kii ALS/PDC) 3
Muscular dystrophy DMD (5), LAMA2 (1), POMT2 (1) 9
Myasthenia Gravis (MG) 1
Myocardial infarction 18
Myoclonic epilepsy CHD2 (1) 1
Myotonic dystrophy CNBP (4), DMPK (1) 9
Nescav syndrome KIF1A (5) 6
Neurodegeneration with brain iron accumulation 5 (NBIA5) WDR45 (1) 1
Neurodevelopmental disorder DHPS (1) 1
Neuroferritinopathy 1
Neurofibromatosis type1 (NF1) 1
Neurofibromatosis type2 (NF2) 1
Neuromyelitis Optica 4
Neuromyelitis Optica Spectrum Disorders (NMOSD) 1
Neuronal migration disorder PIK3R2 (1) 3
Neuropathy GARS (2), SCN9A (6) 39
Niemann-pick disease NPC1 (1), SMPD1 (3) 5
Non-als motor neurone disease 1
Ohtahara syndrome STXBP1 (1) 1
Ornithine transcarbamylase deficiency (OTCD) 1
Osteogenesis imperfecta type iv (OI4) COL1A2 (1) 1
PACS1 (Schuurs-Hoeijmakers) syndrome PACS1 (2) 2
Pain agnosia SCN11A (2) 2
Parkinsonism GBA (2), LRRK2 (6), MAPT (1), PARK2 (4), PINK1 (1), SNCA (3) 26
Parkinson’s disease (PD) GBA (18), LRRK2 (8), SNCA (9) 99
Paroxysmal nocturnal hemoglobinuria (PNH) 1
Pemphigoid (including epidermolysis bullosa acquisita) 2
Pemphigus 2
Periodic paralysis 1
Phenylketonuria PAH PAH (1) 2
Pick's disease 1
Pitt-hopkins syndrome (PTHS) TCF4 (1) 1
Polyarteritis nodosa (PAN) 2
Polymicrogyria 1
Pompe’s disease (adult type) 1
Primary antiphospholipid syndrome 2
Primary erythromelalgia SCN9A (2) 4
Primary immunodeficiency syndrome 3
Primary lateral sclerosis (PLS) 7
Primary open angle (POAG) 20
Primary progressive aphasia (PPA) 1
Progressive multifocal leukoencephalopathy (PML) 1
Progressive supranuclea palsy (PSP) 1
Prolonged QT interval 6
Pulmonary arterial hypertension ALK1 (2), BMPR2 (4) 6
Pulmonary atresia 1
Pustular psoriasis 1
Pyogenic sterile arthritis / Pyoderma gangrenosum and acne syndrome 1
Rasmussen encephalitis 2
Relapsing polychondritis (RP) 1
Resolved systolic anterior motion 1
Restrictive cardiomyopathy 1
Retinitis pigmentosa 5
Rett syndrome FOXG1 (5), MECP2 (6), SHANK3 (1) 15
Right ventricular outflow tract premature ventricular contractions 2
Ring chromosome 20 syndrome 2
Sanfilippo syndrome / MPS IIIC (acetyl-CoA:heparan-α-D-glucosaminide N-acetyltransferase deficiency) 1
Schizophrenia 4
Semantic Dementia 2
Severe combined immunodeficiency ADA (2) 2
Sickle cell anemia HBB (55) 55
Sjögren’s syndrome 1
Skeletal displasia 4
Small atrial septal defect 1
Smith-magenis syndrome (SMS) 1
Spinal muscular atrophy SMN1 (14) 18
Spinal-Bulbar Muscular Atrophy (SBMA) 10
Spinocerebellar Degeneration 14
Spinocerebellar ataxia type 1 2
Spinocerebellar ataxia type 3 ATXN3 (4) 4
Spondylometaphyseal displasia 2
Stevens-Johnson syndrome (SJS) 1
Sturge-Weber syndrome 1
Subacute sclerosing panencephalitis (SSPE) 2
Syringomyelia 1
Systemic amyloidosis 2
TNF receptor-associated periodic syndrome 1
Takayasu arteritis 3
Tangier disease ABC1 (2), ABCA1 (4) 6
Tay-sachs disease (TSD) HEXA (1) 1
Thrombotic thrombocytopenic purpura (TTP) 1
Tricuspid atresia 1
Tuberous sclerosis TSC2 (2) 3
Ventricular tachycardia 5
Vertebrobasilar insufficiency(VBI) 1
Vici syndrome (VICIS) EPG5 (1) 1
Werner syndrome 1
West syndrome 1
Wilson’s disease 4
Wolfram syndrome 1
Wolman disease 1
X-linked creatine transporter deficiency 1
X-linked dystonia Parkinsonism TAF1 VARIANT (34) 34
Xeroderma pigmentosum 2

