Baltimore, Maryland, USA (March 12, 2024) – Elixirgen Scientific, Inc., a biotechnology leader focused on advancing drug discovery and revolutionizing cell therapy through iPSC technology, announced today that it has been accredited for ISO 9001:2015 certification. The certification is an internationally recognized standard developed by the International Organization for Standardization (ISO) that ensures a company’s products and services meet the needs of customers through an effective Quality Management System. This achievement highlights Elixirgen Scientific’s on-going efforts in quality process enhancement across all operations, effective risk management, continuous improvement, and adherence with strict regulatory requirements. The ISO 9001:2015 certification ensures that their iPSC-derived cells and services are developed and delivered under the highest standards of quality management, significantly enhancing their reliability and efficacy for advanced research and therapeutic applications.
The ISO 9001 certification recognizes Elixirgen Scientific’s unwavering commitment to customer satisfaction, exceptional products and services, and maintenance of a robust quality management system to confirm its position as a pioneer in biotechnology. Elixirgen Scientific’s innovative solutions, such as our Quick-Glia™ iPSC-derived astrocytes and our custom differentiation services, aim to improve research into neuroinflammatory and neurodegenerative diseases by enhancing the relevance and efficiency of drug development processes.
Elixirgen Scientific, Inc. underwent an audit that thoroughly reviewed the entire product lifecycle management process, services and product design, and development practices. The initial audit was carried out by QAS International, a renowned independent certification authority. This comprehensive evaluation culminated in the successful issuance of its ISO 9001:2015 certification. QAS International will conduct continuous surveillance audits of their Quality Management System to ensure compliance with ISO 9001 standards and the implementation of best practices for continuous enhancement of their products, services, and customer satisfaction.
"Today's achievement is a significant milestone for us and a testament to our team's relentless pursuit of excellence in iPSC technology and quality management. It reinforces our commitment to leading the way in scientific innovation, backed by the highest standards of quality."
Keiki Sugimoto, Ph.D.
Chief Executive Officer
Elixirgen Scientific, Inc.
For our valued clients, this certification ensures that Elixirgen Scientific’s quality management system, manufacturing processes, and customer service consistently meet ISO’s internationally recognized standards. This achievement enhances our product and service quality, boosts customer satisfaction, and optimizes our business processes, reaffirming our commitment to excellence and innovation.
About Elixirgen Scientific
Elixirgen Scientific, headquartered in the Science + Technology Park at Johns Hopkins in Baltimore, Maryland, is at the forefront of leveraging stem cell-based technologies to accelerate scientific discovery and the development of cures for a broad spectrum of diseases. Their products and services, including the revolutionary Quick-Tissue™ and Quick-Glia™ series, have positioned them as a premier partner in the global scientific community, committed to advancing human health through scientific excellence.
For Further Information, Contact:
Fujisawa, Japan (December 07, 2023) – Elixirgen Scientific Japan, Inc (EsJ), a Japanese provider of synthetic mRNA contract development and manufacturing services, was awarded TOP 10 CDMOs in APAC - 2023 by Pharma Tech Outlook.
Recognized for its excellence in delivering innovative and high-quality solutions in mRNA manufacturing, EsJ has demonstrated significant advancements in this field since its inception of CDMO (Contract Development and Manufacturing Organization) services in September 2021. These advancements have played a pivotal role in achieving this prestigious recognition. By providing high-quality mRNA, we will support pharmaceutical industries to accelerate R&D for mRNA medicine such as vaccines.
This esteemed recognition by Pharma Tech Outlook is based on several key factors, including EsJ's state-of-the-art technology, commitment to quality, and impactful contributions to the pharmaceutical industry. The award highlights EsJ's efforts in enhancing the efficiency and scalability of mRNA manufacturing processes, making significant strides in the field of biotechnology.
TOP 10 CDMOs in APAC - 2023 by Pharma Tech Outlook
About Elixirgen Scientific Japan
Elixirgen Scientific Japan is a biotechnology company engaged in contract manufacturing of synthetic mRNA and stem cell-related products and services. Based on its technology for synthesizing mRNA in a cGMP-compliant environment, which is the standard for manufacturing and quality control of pharmaceutical products, and its technology for reproducibly inducing differentiation from iPS cells into various types of cells in about one week, the company aims to improve the quality of people's lives by supporting faster and lower-cost drug discovery. With a vision to revolutionize the field of regenerative medicine and drug development, EsJ continues to innovate and expand its capabilities, aspiring to be at the forefront of biotechnological and pharmaceutical advancements.
For more information, contact [email protected].
Science and Technology Park at Johns Hopkins
855 N. Wolfe St., Suite 631
Baltimore, MD 21205
HOURS:
MONDAY-FRIDAY 9AM-5PM EST
TriLink CleanCap® mRNA capping technology now accessible to ESJ customers for non-commercial use
FUJISAWA (SEPTEMBER 26, 2023) – TriLink BioTechnologies (TriLink®), a Maravai™ LifeSciences company (NASDAQ: MRVI) and global provider of life science reagents and services, has entered into a non-exclusive License and Supply Agreement for TriLink’s CleanCap® mRNA capping technology with Elixirgen Scientific Japan, Inc (ESJ), a Japanese provider of synthetic mRNA contract development and manufacturing services.
Aligned to the terms of the agreement, TriLink® will supply its proprietary CleanCap® M6, CleanCap® AG 3’Ome, and CleanCap® AG cap analogs for use in ESJ’s mRNA development and manufacturing services, from pre-clinical through Phase III programs.
This agreement aligns with TriLink’s objective to enable greater access to CleanCap mRNA capping technologies. CleanCap technology produces an optimal Cap1 structure with over 95% efficiency, creating a one-pot solution that can help cut production processes by one week, and reduce overall manufacturing costs by 20-40% when compared to other capping methods.
“Our novel capping solutions improve mRNA function, help streamline manufacturing processes and maximize capped material yield, accelerating customer discoveries,” shared Drew Burch, EVP of TriLink’s Nucleic Acid Products division.
Since the launch of the first CleanCap analog in 2017, its technology continues to advance the mRNA capping industry and was used in one of the first commercially approved COVID-19 vaccines. Additionally, TriLink continues to evolve and expand its innovation with the launch of the CleanCap M6 analog, the most robust analog to date, with studies indicating increased mRNA expression by more than 30% versus enzymatic capping method.
“Having the opportunity to offer our customers TriLink’s industry-leading platforms and technology is pivotal,” shared Motoki Azuma, PhD, President of ESJ. “This access to premier reagents and other technology will fuel our customers’ R&D to support the growth of the industry at large.”
*Data and customer insights garnered from a market assessment conducted by a third-party consulting firm on behalf of TriLink in November 2022.
About TriLink BioTechnologies
TriLink BioTechnologies, a Maravai LifeSciences company, is helping to realize the power and potential of mRNA. As a global leader in nucleic acid and mRNA solutions for more than 25 years, TriLink delivers unrivaled chemical and biological experience, CDMO services, and high-quality readymade and custom materials, including its proprietary CleanCap® mRNA capping technology. Pharmaceutical leaders, biotech disruptors and world governments depend on TriLink to meet their greatest challenges, from delivering the COVID-19 vaccine at warp speed, to empowering innovative treatments in oncology, infectious diseases, cardiology, and neurological disorders, to enabling future pandemic response plans.
For more information, visit trilinkbiotech.com
About Maravai LifeSciences
Maravai is a leading life sciences company providing critical products to enable the development of drug therapies, diagnostics, and novel vaccines. Maravai’s companies are leaders in providing products and services in the fields of nucleic acid synthesis and biologics safety testing to many of the world’s leading biopharmaceutical, vaccine, diagnostics, and cell and gene therapy companies.
For more information about Maravai LifeSciences, visit maravai.com
About Elixirgen Scientific Japan
Elixirgen Scientific Japan is a biotechnology company engaged in contract manufacturing of synthetic mRNA and stem cell-related products and services. Based on its technology for synthesizing mRNA in a cGMP-compliant environment, which is the standard for manufacturing and quality control of pharmaceutical products, and its technology for reproducibly inducing differentiation from iPS cells into various types of cells in about one week, the company aims to improve the quality of people's lives by supporting faster and lower-cost drug discovery.
For more information, contact [email protected]
A joint research of Ricoh and the University of Tokyo
TOKYO, March 27, 2023 – Ricoh's research group at the Biomedical R&D Department, led by Waka Lin, and Yuko Sekino, Project Professor at the Graduate School of Agriculture and Life Sciences, the University of Tokyo, have jointly demonstrated that transcription factor-induced human iPSC-derived neurons reach functional maturity by rapidly achieving the formation of dendritic spines and the expression of mechanisms underlying synaptic plasticity. The final version of the research article is available online at the American scientific journal
iScience, updated March 23, 2023.
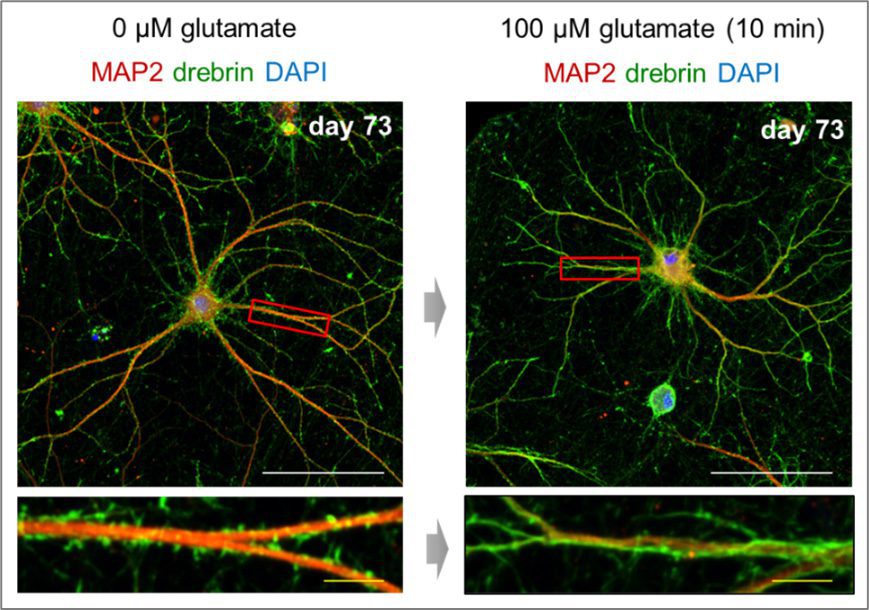
Dendritic spine formation and drebrin exodus in iPSC-derived neurons
Left: visualization of mature dendrites (red) and spines (green) at day 73 of culture after differentiation
Right: drebrin exits from the spines heads upon glutamate stimulation
(Scale bars: White = 100 µm. Yellow = 10 µm)
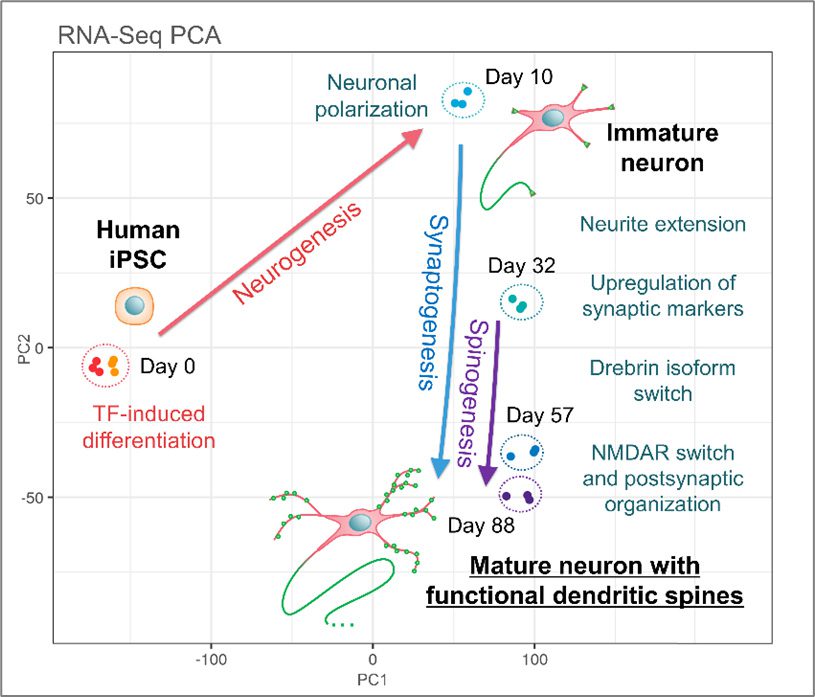
Graphical abstract summarizing the spine development and synaptic maturation process of transcription factor-induced iPSC-derived neurons
The highlights and outline of the research findings are as follows:
Highlights:
- Researchers have demonstrated that transcription factor-induced iPSC-derived neurons reproduced the maturation process of human brain neurons and achieved a highly efficient formation of dendritic spines.
- Successful synaptic maturation allowed the observation of drebrin exodus, a cellular mechanism underlying learning and memory, for the first time in human iPSC-derived neurons.
- The mature iPSC-derived neurons generated in this study are expected to facilitate research on human synaptic functions and drug development targeting cognitive disorders.
Outline:
After induction of differentiation using a transcription factor (Note 1)-based method from Elixirgen Scientific, Inc., iPSC-derived neurons (Note 2, 3) displayed numerous dendritic spines (Note 4) in a relatively short culture time of 2 to 3 months. Time-dependent changes in gene expression patterns correlated with human brain development data and showed characteristic features of postnatal maturation, such as the conversion of drebrin (Note 5) to its brain-specific isoform (Note 6) drebrin A. Moreover, the study revealed for the first time in human iPSC neurons the conservation of the cellular event known as drebrin exodus, wherein drebrin accumulated in the spine heads migrates into dendritic shafts in response to glutamate stimulation. Drebrin exodus was previously reported to be involved in the spine structural plasticity (Note 7) of rodent primary neurons. Its observation in human neurons is of great significance for facilitating research on the maturation of human synapses and on mechanisms underlying learning and memory. Additionally, the time required for the formation of dendritic spines was reduced by one-third in transcription factor-induced iPSC neurons, which may help to reduce experimental costs substantially.
These results hold promise for a better understanding of central nervous system diseases and drug development targeting cognitive disorders. Moreover, the new opportunities raised by the availability of functionally mature human neurons would promote the iPS cell industry and the development of pharmaceutical applications, including in vitro assays for drug safety and toxicity testing.
Paper Information:
- Journal: iScience
- Title: Dendritic spine formation and synapse maturation in transcription factor-induced human iPSC-derived neurons
- Authors: Waka Lin*, Shusaku Shiomoto, Saki Yamada, Hikaru Watanabe, Yudai Kawashima, Yuichi Eguchi, Koichi Muramatsu, and Yuko Sekino
- DOI: 10.1016/j.isci.2023.106285
- URL: https://doi.org/10.1016/j.isci.2023.106285
Glossary:
- (Note 1)
- Transcription factor: A protein that initiates or regulates mRNA expression by transcribing genetic information from genomic DNA. The transcription factors expressed in a cell determine its properties and behavior.
- (Note 2)
- iPSC-derived neurons: Neurons artificially generated in vitro by differentiating iPS cells (Note 3).
- (Note 3)
- Induced pluripotent stem cells (iPS cells): Cells generated from somatic cells, such as human skin and blood cells, by introducing specific reprogramming factors, so that they acquire the ability to differentiate into any type of cell in the body.
- (Note 4)
- Dendritic spines: Human and animal brain neurons form neuronal networks with axons that transmit information from one cell to another and dendrites that receive the information. Dendrites have thousands of microscopic protrusions called spines that receive signal inputs from axon terminals. Dendritic spines contain various proteins involved in memory and learning.
- (Note 5)
- Drebrin: An actin-binding protein that stabilizes the actin fiber cytoskeleton controlling cellular morphology. The brain-specific isoform drebrin A accumulates at the postsynaptic sites and regulates the formation and dynamics of dendritic spines. Drebrin is known to exit the dendritic spines and migrate into dendritic shafts in response to excitatory stimulation, such as exposure to glutamate. This cellular mechanism, known as drebrin exodus, is essential for the induction of structural plasticity (note 7).
- (Note 6)
- Isoform: When a single gene produces multiple proteins, each alternative protein form is referred as an isoform. One isoform may be replaced by another under specific conditions. For example, drebrin is expressed by a single gene (DBN1), but the embryonic isoform drebrin E is converted to the brain-specific isoform drebrin A during neural maturation.
- (Note 7)
- Structural plasticity: Changes in spine shape and volume are associated with long-term changes in synaptic transmission efficiency underlying memory formation. Structural plasticity plays a crucial role in learning and forgetting mechanisms.
TOKYO, May 17, 2022 – Ricoh Company, Ltd. and Elixirgen Scientific, Inc. (Baltimore, Maryland, USA) announce that they have agreed and signed a contract for Ricoh to acquire a majority stake in Elixirgen Scientific. Ricoh had previously acquired 34.5% of Elixirgen Scientific's stock in 2019. By having Elixirgen Scientific as its subsidiary, Ricoh aims to contribute to human health and security by accelerating the development and construction of drug discovery infrastructure to solve social issues such as aging and pandemics.
Elixirgen Scientific contributes to the efficiency of drug discovery research and disease research using iPS cells with its proprietary "Quick-Tissue™" technology that enables high-speed differentiation*1 of iPS cells*2 and ES cells (embryonic stem cells) into various types of cells. Ricoh will expand the application of Elixirgen Scientific's technology and contribute to the acceleration of personalized medicine*3 and drug discovery research with its digitalization and Artificial Intelligence (AI) technologies. In addition, by utilizing Elixirgen Scientific's abundant cell experiment data, the two companies aim to start a business in predicting drug responses and disease mechanisms using AI technology.
In September 2021, Elixirgen Scientific became the first company in the Asia-Pacific region** to launch a CDMO business (contract development and manufacturing of pharmaceutical products) for mRNA*4 drugs. In November of the same year, it established a Japanese subsidiary, Elixirgen Scientific Japan, to strengthen its business in Japan. Ricoh will support the company's business with its automation technology and production management know-how to expand the scale and efficiency of the CDMO business. By strengthening Elixirgen Scientific’s medical mRNA production capacity in the region, Ricoh will support the production of vaccines and other mRNA investigational drugs and mRNA active pharmaceutical ingredients.
** Based on research by Elixirgen Scientific, as of September, 2021.
Overview of Drug discovery support using mRNA and iPS cells
As new infectious diseases and the aging of the population continue to advance worldwide, personalized medicine is expected to become a reality. On the other hand, shortening the development period for the production of candidate substances, selection of substances with medicinal properties, and safety verification has become an important issue in researching and developing new drugs required for personalized medicine.
As the rapid commercialization of vaccines for COVID-19 has attracted attention, drug discovery using mRNA can significantly shorten the research and development period compared to conventional pharmaceuticals. This is because it is possible to design effective sequences in a short period by, for example, copying a part of specific genetic information. It is expected that this technology will be utilized in vaccines and cancer drugs.
By differentiating iPS cells into a variety of cell types, iPS cells can be used to pre-confirm the effects of candidate drugs on patients with various genetic backgrounds. As a result, candidate drugs for animal experiments and clinical trials can be narrowed down quickly and efficiently.
*1 iPS cells: Induced pluripotent stem cells. Pluripotent stem cells are artificially created by culturing cells and are capable of differentiating into various types of cells.
*2 Differentiation: The process of creating target cells from iPS cells and ES cells.
*3 Personalized medicine: The optimal treatment according to the condition of the disease and the constitution of the individual (genetic information, etc.).
*4 mRNA: Messenger RNA (Ribonucleic acid): RNA that copies part of the genetic information from DNA and synthesizes proteins.
Related News
Ricoh Announces Strategic Business Partnership with Elixirgen Scientific
https://www.ricoh.com/release/2019/0619_1
Offering assay-ready multi-electrode array plates to measure electrical activities of human iPS cell-derived neurons
https://www.ricoh.com/release/2020/1203_1
For further information, please contact:
Ricoh Company, Ltd. www.ricoh.com
Public Relations TEL:050-3814-2806 E-mail: [email protected]
Biomedical Business Group E-mail: [email protected]
Elixirgen Scientific, Inc. https://www.elixirgensci.com/
E-mail: [email protected]
About Elixirgen Scientific
Elixirgen Scientific is a Baltimore, Maryland-based biotechnology company engaged in the research, development, manufacture, and sale of stem cell-related products and contract manufacturing of synthetic mRNA (established Elixirgen Scientific Japan in November 2021). The company is a biotech company.
Based on its technology for reproducibly inducing differentiation from iPS cells into various types of cells in about one week and its technology for synthesizing mRNA in a cGMP-compliant environment, which is the standard for manufacturing and quality control of pharmaceutical products, the company aims to improve the quality of people's lives by supporting faster and lower-cost drug discovery.
For further information, please visit www.elixirgensci.com
About Ricoh
Ricoh is empowering digital workplaces using innovative technologies and services that enable individuals to work smarter from anywhere.
With cultivated knowledge and organizational capabilities nurtured over its 85-year history, Ricoh is a leading provider of digital services, information management, and print and imaging solutions designed to support digital transformation and optimize business performance.
Headquartered in Tokyo, Ricoh Group has major operations throughout the world and its products and services now reach customers in approximately 200 countries and regions. In the financial year ended March 2022, Ricoh Group had worldwide sales of 1,758 billion yen (approx. 14.5 billion USD).
For further information, please visit www.ricoh.com
We are excited to announce that Swift Scientific will now be Elixirgen Scientific’s sales representative in the Northeastern US territory. Together we anticipate business expansion with wider support in the region to better serve our valued clients.
Quick-Neuron™ Precursor differentiated cell cultures continue to proliferate and express a variety of NPC markers, such as nestin (NES), vimentin (VIM), Hes Family BHLH Transcription Factor 5 (HES5), SRY-Box Transcription Factor 1 (SOX1), and SRY-Box Transcription Factor 2 (SOX2).
- Differentiation achieved in just 6 days
- Proprietary transcription factor-based stem cell differentiation
- No genetic footprint
- Viable long-term
- Suitable for a variety of characterization and differentiation
Our Quick-Neuron™ Sensory differentiated cell cultures display typical neurite outgrowth and express a variety of neuronal markers, such as tubulin beta 3 class III (TUBB3) and variety of sensory neuron markers such as peripherin (PRPH), islet-1 (ISL1), and brain-specific homeobox/POU domain protein 3A (BRN3A/POU4F1).
• Differentiation achieved in just 10 days • Proprietary transcription factor-based stem cell differentiation • No genetic footprint • Viable long-term • Suitable for a variety of characterization and neurotoxicity assays
Our Quick-Neuron™ Precursor - Human iPSC-derived Neural Precursor Cells (NPCs) proliferate and express a variety of NPC markers, such as neuroectodermal stem cell marker, Nestin (NES) and Vimentin (VIM).
• Available from multiple healthy and patient iPSC lines
• Proprietary transcription factor-based stem cell differentiation
• No genetic footprint
• Viable long-term
• Suitable for characterization and further differentiation
• Available in two sizes (1 million or 5 million viable cryopreserved cells)
The Quick-Glia™ Astrocyte - SeV Kit facilitates rapid and efficient differentiation of human iPS or ES cells into astrocyte cells. Quick-Glia™ Astrocyte differentiated cell cultures display typical astrocyte morphology and markers such as S100 Calcium Binding Protein ß (S100ß), Chondroitin Sulfate Proteoglycan 8 (CD44), Aldehyde Dehydrogenase 1 Family Member L1 (ALDH1L1), and mature astrocyte marker Glial Fibrillary Acidic Protein (GFAP).
• Differentiation achieved in just 28 days
• Proprietary transcription factor-based stem cell differentiation
• No genetic footprint
• Viable long-term
• Suitable for a variety of characterization and assays



